MS Medium Preparation Guide
Murashige and Skoog (MS) medium is the most widely used plant tissue culture medium in modern plant biotechnology. Originally formulated in 1962 by Toshio Murashige and Folke Skoog, it was designed to support rapid cell division and organogenesis in tobacco tissue cultures. Today, MS medium is considered the gold standard for a wide range of plant species and applications.
Materials & Equipment
Bramble Cay MS medium powder |
Distilled / deionized water.png) |
Sucrose
|
Agar or gelling agent
|
pH meter |
Magnetic stirrer & hot plate |
Autoclave.png) |
Culture vessels / containers |
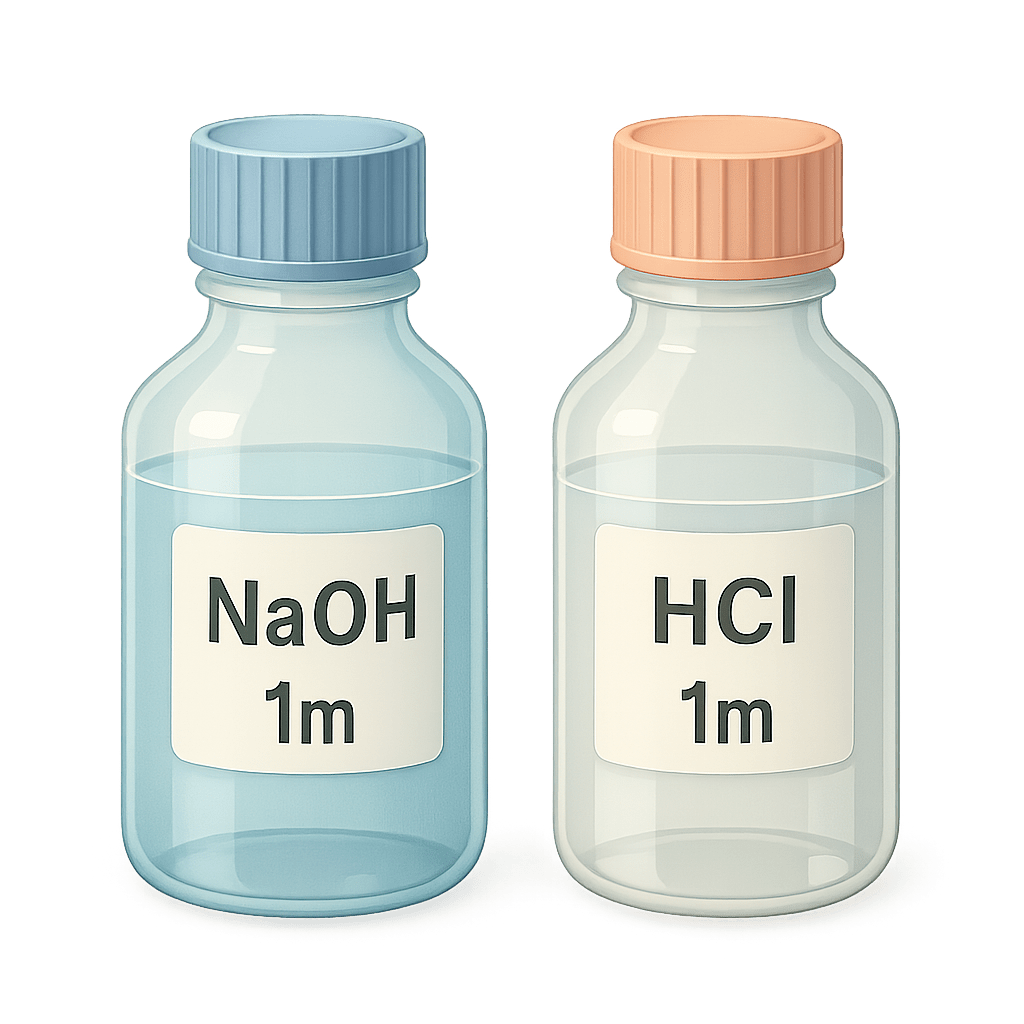
Dissolve Basal Salts
Gently stir 4.33 g Bramble Cay MS powder with ~800 ml distilled water until completely dissolved.

The product label usually provides standard recommendations for use. If a different concentration is required, the following parameters may be used.
Murashige and Skoog (MS) basal medium concentrations (per 1 liter):
- MS (1×): 4.33 g per liter
- MS (½ MS): 2.17 g per liter
- MS (⅓ MS): 1.44 g per liter
- MS (¼ MS): 1.08 g per liter



Add Sucrose
Mix in 30 g/L sucrose. Stir well for a uniformly clear solution.



.webp)
Make Up Volume
Add distilled water to a total volume of 1 liter and mix thoroughly.

Adjust pH
Use a pH meter and 1 N NaOH or HCl to adjust the pH to 5.6–5.8.

Add Gelling Agent
Add 7–8 g/L agar (or gellan). Stir until the medium is homogeneous.
.webp)



Dispense Medium
Once sucrose and the gelling agent have been added, you have two common options:
Option A — Heat first, then dispense, then autoclave (recommended when working in a flow box or without a true laminar cabinet)
Gently heat the medium in a microwave without bringing it to a boil, until the agar is fully dissolved and the solution becomes completely homogeneous. Dispense the hot medium into culture vessels, leaving headspace for expansion, then autoclave the filled vessels to sterilize the medium.
Option B — Autoclave first, then dispense aseptically (only if your laminar flow cabinet provides sufficiently sterile airflow)
Autoclave the medium in a suitable autoclavable bottle or flask, allow it to cool to a safe handling temperature, then dispense it inside a laminar flow cabinet into pre-sterilized culture vessels. This option is suitable only when the laminar cabinet provides reliable air sterility. If you do not have a laminar cabinet and are working in a flow box, we recommend Option A, as it typically helps reduce contamination rates.
WARNING
Working with an autoclave involves certain risks. Carefully ensure that the lids of all containers are not tightly sealed, as this may cause pressure buildup leading to container rupture, explosion, and serious injury. Always follow proper safety procedures when operating equipment that uses pressurized superheated steam, and make sure you are familiar with the relevant autoclave safety guidelines before use.
Sterilize
Autoclave at 121 °C for 15–20 minutes to sterilize the medium.



Cool & Finalize
Allow to cool; if necessary, add heat-sensitive supplements at 45–50 °C under sterile conditions.
Tips for Best Results
- Dissolve all salts fully before adjusting pH.
- Avoid overheating agar or sugar to prevent caramelization.
- Weigh gelling agents accurately for consistent texture.
- Store ready medium at 4 °C for up to 4 weeks.
- Use half-strength MS for sensitive cultivars or seeds.
- Add activated carbon if needed for phenolic secretions.
We are deeply grateful for choosing Bramble Cay products.
This guide was created with care to help you achieve the best results with your plant tissue culture work. If you have any questions or need further assistance, our support team is always here for you — don’t hesitate to reach out. Together, we grow better.
If you have any questions, feel free to ask in our Telegram community: t.me/tissue_culture_club_chat